
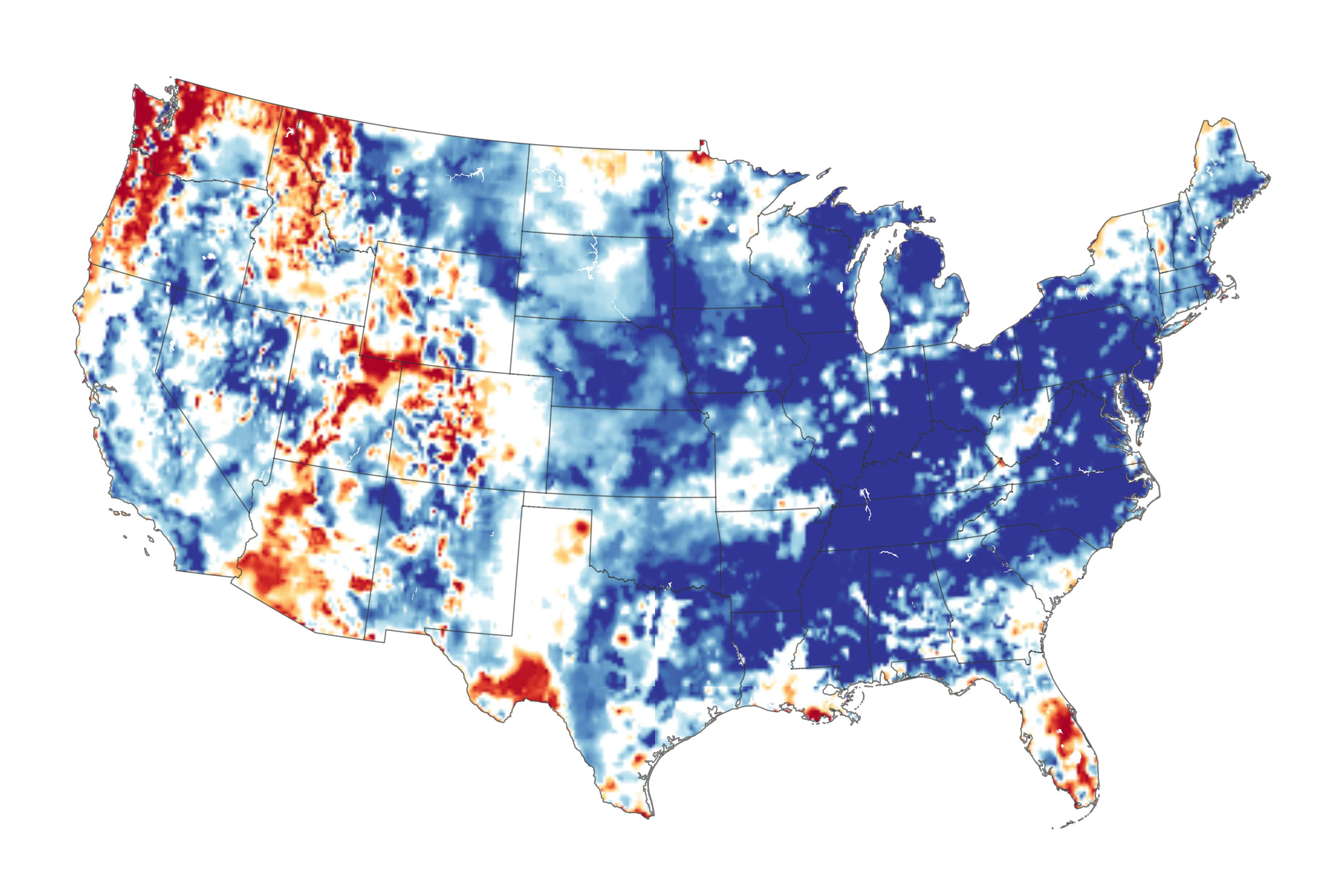
The top image shows AgBr before exposure to light, and the bottom image after exposure.Īfter the film is developed, any unexposed silver bromide must be removed by a process called “fixing” otherwise, the entire film would turn black with additional exposure to light. 2PO 4 3−(aq) + 3Hg 2+(aq) → Hg 3(PO 4) 2(s)ĭarkening of silver bromide crystals by exposure to light.Aqueous solutions of calcium bromide and cesium carbonate are mixed.Solid sodium fluoride is added to an aqueous solution of ammonium formate.Solid potassium phosphate is added to an aqueous solution of mercury(II) perchlorate.An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(II) chloride.The net ionic equation is as follows: Pb 2+ (aq) + 2I −(aq) → PbI 2(s) Thus Pb(C 2H 3O 2) 2 will dissolve, and PbI 2 will precipitate. The only possible exchange reaction is to form LiCl and BaSO 4:ī According to Table 4.2 "Guidelines for Predicting the Solubility of Ionic Compounds in Water", ammonium acetate is soluble (rules 1 and 3), but PbI 2 is insoluble (rule 4). Mixing the two solutions initially gives an aqueous solution that contains Ba 2+, Cl −, Li +, and SO 4 2− ions. If a precipitate forms, write the net ionic equation for the reaction.Ī Because barium chloride and lithium sulfate are strong electrolytes, each dissociates completely in water to give a solution that contains the constituent anions and cations. Solid lead(II) acetate is added to an aqueous solution of ammonium iodide.Īsked for: reaction and net ionic equationĪ Identify the ions present in solution and write the products of each possible exchange reaction.ī Refer to Table 4.2 "Guidelines for Predicting the Solubility of Ionic Compounds in Water" to determine which, if any, of the products is insoluble and will therefore form a precipitate.Aqueous solutions of strontium bromide and aluminum nitrate are mixed.Aqueous solutions of rubidium hydroxide and cobalt(II) chloride are mixed.Aqueous solutions of barium chloride and lithium sulfate are mixed.Write the net ionic equation for any reaction that occurs. Using the information in Table 4.2 "Guidelines for Predicting the Solubility of Ionic Compounds in Water", predict what will happen in each case involving strong electrolytes. When these solutions are mixed, the only effect is to dilute each solution with the other ( Figure 4.12 "The Effect of Mixing Aqueous KBr and NaCl Solutions"). As you will see in the following sections, none of these species reacts with any of the others. For example, if 500 mL of a 1.0 M aqueous NaCl solution is mixed with 500 mL of a 1.0 M aqueous KBr solution, the final solution has a volume of 1.00 L and contains 0.50 M Na +(aq), 0.50 M Cl −(aq), 0.50 M K +(aq), and 0.50 M Br −(aq).

Simply mixing solutions of two different chemical substances does not guarantee that a reaction will take place. Just as important as predicting the product of a reaction is knowing when a chemical reaction will not occur. Salts of +1 cations, Mg 2+, and dipositive transition metal cations (e.g., Ni 2+)
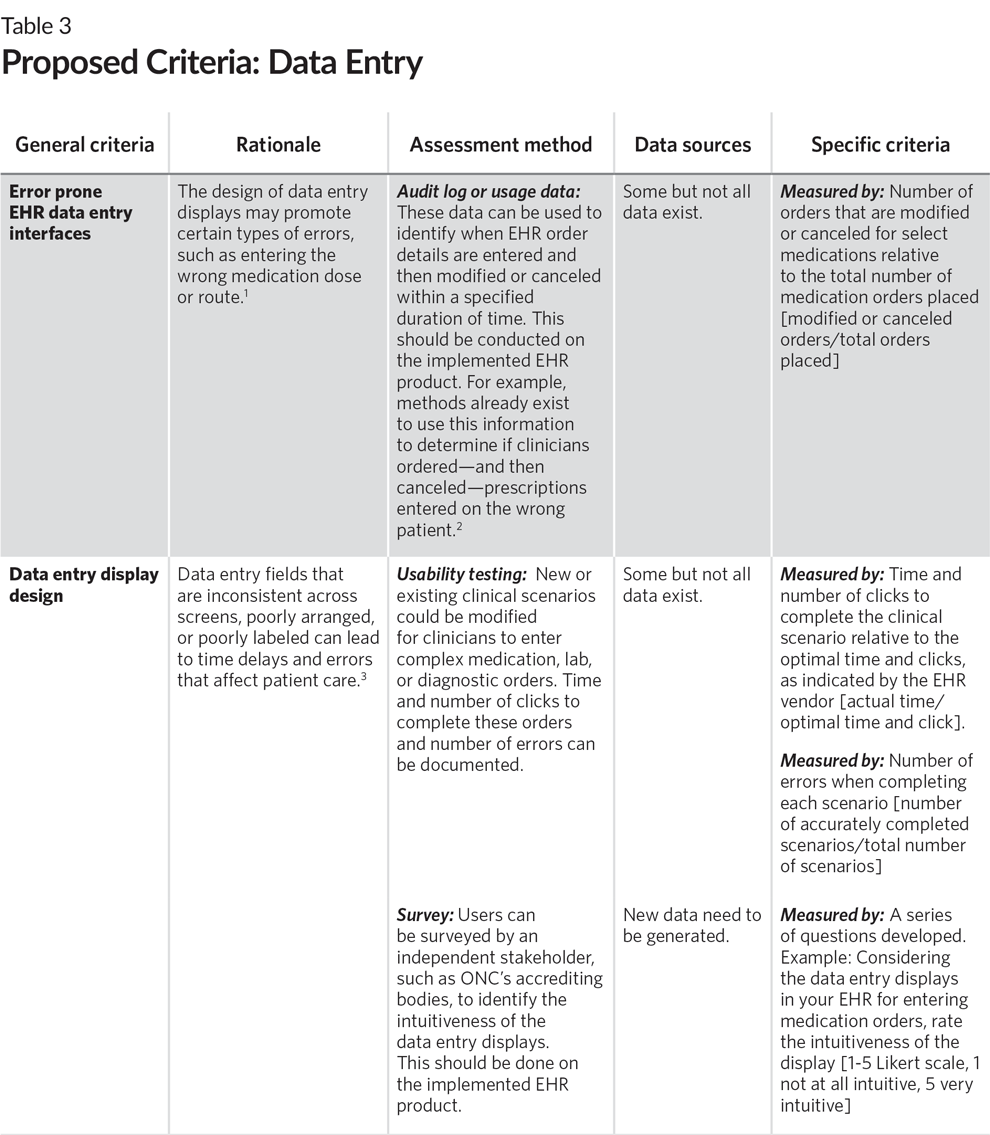
Most sulfate (SO 4 2−) salts that contain main group cations with a charge ≥ +2 Salts of the alkali metals or the NH 4 + ion. Most carbonate (CO 3 2−) and phosphate (PO 4 3−) salts Salts of the alkali metals (group 1), the heavier alkaline earths (Ca 2+, Sr 2+, and Ba 2+ in group 2), and the NH 4 + ion. Most salts that contain the hydroxide (OH −) and sulfide (S 2−) anions Salts of metal ions located on the lower right side of the periodic table (e.g., Cu +, Ag +, Pb 2+, and Hg 2 2+). Silver acetate and salts of long-chain carboxylates Most salts of anions derived from monocarboxylic acids (e.g., CH 3CO 2 −) Most salts that contain the nitrate (NO 3 −) anion Most salts that contain an alkali metal (Li +, Na +, K +, Rb +, and Cs +) and ammonium (NH 4 +) Compare recording-setting values to their previous values.Table 4.2 Guidelines for Predicting the Solubility of Ionic Compounds in Water


 0 kommentar(er)
0 kommentar(er)
